T-DM1
Fewer agents raise my eyebrow now that every new cancer
drugs is labeled as some form of a “nib” or a “mab.” All such categories of
targeted antibody to a defined mutated receptor, an epigenetic related loss or
gain of function of a gene, or specific protein product of a gene-gone-haywire
therapy assault the surface antigen of the cell where the “nib” or “mab” meet
the cell and prevent the flow of a
signal from reaching the nucleus (interior) of the cell and thus preventing its
(the cells) reduplication.
Cellular Internalization of the T-DM1 molecule
Signal Transduction:
The signal, you see, is the message from the outside (the exterior of the cell wall) that tells the interior (nuclear) machinery to go into action and replicate another one like itself. On the other hand, the unmitigated growth of the cancer cell is reliant on promoting that signal in a loop mechanism and by virtue of that and the multiple transduction pathways, this leads to the constant barrage of signals to the nucleus giving the continuous directive to grow.
The signal, you see, is the message from the outside (the exterior of the cell wall) that tells the interior (nuclear) machinery to go into action and replicate another one like itself. On the other hand, the unmitigated growth of the cancer cell is reliant on promoting that signal in a loop mechanism and by virtue of that and the multiple transduction pathways, this leads to the constant barrage of signals to the nucleus giving the continuous directive to grow.
The intracellular communication:
Consider a branching railway line all merging into a single line as so happens at Railway Junctions. If one line goes bad at the junction, it can be piggy-backed/connected to another track or at the station the passengers can board another train that can be programmed for similar destination. But once the rail lines merge into one, there is only one way out of the station. We are inching our way to disrupting that single railway line.
Consider a branching railway line all merging into a single line as so happens at Railway Junctions. If one line goes bad at the junction, it can be piggy-backed/connected to another track or at the station the passengers can board another train that can be programmed for similar destination. But once the rail lines merge into one, there is only one way out of the station. We are inching our way to disrupting that single railway line.
At present disrupting the pre-merged railway lines just invites the train to
take another line enroute to the common outbound line. That is the reason for
the many targeted therapies to have a moment of success in sunshine with cancer but they ultimately fail to maintain the momentum
.
.
T-DM1
So what caught my eye was this new drug called T-DM1.
Fascinating name to begin with, this drug inspired a closer look. The “T” in
the T-DM1 stands for the Trastuzumab as in Herceptin. Trastuzumab is directed at the HER 2 receptors (The surface
antigens I mentioned earlier) present on the breast and various other cancer
cells. So this drug interferes
with the signal from the surface (mediated via the HER-2 receptor) to the
interior of the cell, required for the cancer cells to grow. The hitch in some cases is the absence of over expression of these receptors on the cancer cell surface of the HER 2 or the Trastuzumab (Monoclonal antibody to HER 2) cannot collocate to the HER-2 receptor to get internalized
and therefore becomes impotent in its action to subvert the HER-2 function from generating the signal.
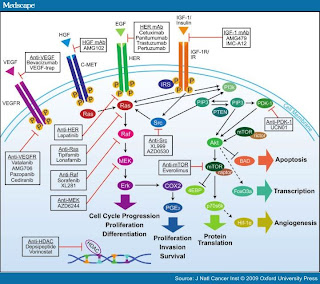
A (very) Few of the Pathways (Medscape)
Additionally,
another mechanism for failure is the cross-talk between the pathways
(interconnecting tracks at the railway junction) merge to allow the signal to be redirected to the interior
of the cell via another pathway and once message is delivered, the cancer cell
resumes its wayward growth (this then also represents the time delay garnered as "Response" to the targeted therapy followed by failure).
You might ask, why did the body do this multiple pathway
“train-station” business inside the cell and make it so difficult to treat? The
reason being that these receptors are needed for the growth of the normal
cells, but in the normal cells the growth is limited to the need and once that
need is satisfied, everything goes back to the quiescent phase of “wait and
watch” demand. In cancer, the cells acquire this innate ability to keep
themselves in business of growth, (a constant state of demand) much like a
politician always promising.
Cancer Cell
So we now get down to the DM1 part. And here is where the
real science behind this drug may be transformational. You see back in the late
1900s, there was a drug called Myatansinoid antimicrotubular agent or DM1((N2′-deacetyl-N2′-(3-mercapto-1-oxopropyl)-maytansine
C35H48ClN3O10S). DM1 is a derivative of the
natural microbial fermentation product and extraordinarily potent
antimicrotubule agent ansamitocin P-3.4 It was first studied back in the
1970s.
T-DM1 attached to HER2 Receptor on Cell Surface
The Chemistry behind:
Chemical derivatization of maytansine resulted in the
synthesis of a maytansine derivative DM1, which is three- to 10-fold more
potent than maytansine, with an inhibitory concentration (IC50) in the
picomolar range and broad cytotoxic activity against a wide range of human
cancers. Maytansine, although active at extremely low concentrations, exhibited
too narrow a therapeutic safety margin, with significant and sometimes
unpredictable gastrointestinal toxic side effects that prevented further
clinical development. The mode of action determined at that time was that the
cleavage between T and DM1 occurs intra-cellularly and also in the plasma, resulting
in the progressive depletion of DM1 molecules eventually yielding the
individual components of unconjugated antibody and “free” DM1.
Mertansine is linked via a complicated structure –
4-(3-mercapto-2,5-dioxo-1-pyrrolidinylmethyl)-cylohexanecarboxylic acid or MCC
–, in which case the International
Nonproprietary Name of the conjugate is formed with emtansine as in
T-DM1.
Antibody Drug Conjugate (ADC):
So T-DM1 is an "Antiibody Drug Conjugate" (ADC) between the Trastuzumab (Herceptin) and
the DM1. The idea behind this immuno-conjugate (ADC) is that the Trastuzumab is
attracted to the HER-2 receptor on the cell surface and binds with it. The
Trastuzumab is “swallowed” (internalized) into the cell cytoplasm where the
delinking between the Trastuzumab and the DM1 occur and then the DM1 is free to
play its role of arresting cell division via the microtubulin assembly (An assembly of microtubules that anchor at polar opposites of a dividing cell to "pull" 50% of the nuclear pieces to form "baby cells") and thus
rendering it to “programmed cell death” or apoptosis. “The trastuzumab
moiety of this ADC binds to HER2 on tumor cell surface surfaces; upon
internalization, the DM1 moiety is released and binds to tubulin, thereby disrupting
microtubule assembly/disassembly dynamics and inhibiting cell division and the
proliferation of cancer cells that overexpress HER2.”
EMELIA Trial:
The benefits of T-DM1 were determined from the EMELIA trial
that suggest a Progression Free Survival (PFS) in the T-DM1 group of patients
compared with the therapy regimen of Capecitabine and Lapatinib. The translated benefit is 3.2 months
(9.6 vs 6.4 months). “Median overall survival for patients treated with
T-DM1 was not reached, but median overall survival for those treated with
standard therapy was 23.3 months (hazard ratio [HR], 0.621; P = .0005)”. Although
this does not seem so grand in scale, these patients have been pretreated
significantly before, prior to being accrued in this study and that is what
gives some solace to the drug designers and those caring for cancer patients
and hopefully, down the line, to the patient’s and their families.
In the EMELIA trial “a total of 978 patients in the
cohort received treatment. The median durations of follow-up were 12.9 months
in the T-DM1 group and 12.4 months in the standard-therapy group. The baseline
demographics, previous therapy, and disease characteristics were balanced
between the 2 groups.”
Outcomes
Measures
|
T-DMI
|
Standard
Therapy
|
Overall
Survival at 1 Year, %
|
84.7
|
77.0
|
Overall
Survival at 2 Years, %
|
65.4
|
47.5
|
Objective
Response Rate, Months (95% CI)
|
43.6
(38.6–48.6)
|
30.8
(26.3–35.7)
|
Duration
of Response in Patients
With
Overall Response, Months
|
12.6
|
6.5
|
Adverse
Events of Grade 3 or Higher, %
|
40.8
|
57.0
|
The drug is by no means a one of a kind. The
immuno-conjugations (ADCs) have been created previously between DM1 and other “mabs;”
Bivatuzumab, Cantuzumab and Lorvutuzumab. The fact that the benefit is modest
with lowered toxicity gives a whole new meaning to immuno-conjugation and the
future of cancer therapy.
Oh and if you are forward thinking, you might ask can this be used in early stage cancer as adjuvant therapy? And the answer is why yes of course, as long as future studies continue to sho robust responses. Why that is how all medications are first tried in advanced stage disease and then showing benefit, they are tailored back in the adjuvant (read preventative) settings.
Will the FDA approve T-DM1? It is not a question of if but when.
Oh and if you are forward thinking, you might ask can this be used in early stage cancer as adjuvant therapy? And the answer is why yes of course, as long as future studies continue to sho robust responses. Why that is how all medications are first tried in advanced stage disease and then showing benefit, they are tailored back in the adjuvant (read preventative) settings.
Will the FDA approve T-DM1? It is not a question of if but when.
So now we know of what is to come in the future.
References:
Chari RV, Martell BA, Gross JL, et al: Immunoconjugates
containing novel maytansinoids: Promising anticancer drugs. Cancer Res
52:127–131, 1992
Chabner BA, Levine AS, Johnson BL, et al: Initial clinical
trials of maytansine, an antitumor plant alkaloid. Cancer Treat Rep 62:429–433,
1978
Blum RH, Kahlert T: Maytansine: A phase I study of an ansa
macrolide with antitumor activity. Cancer Treat Rep 62:435–438, 1978
Cabanillas F, Rodriguez V, Hall SW, et al: Phase I study of
maytansine using a 3-day schedule. Cancer Treat Rep 62:425–428, 1978
Eagan RT, Ingle JN, Rubin J, et al: Early clinical study of
an intermittent schedule for maytansine (NSC-153858): Brief communication. J
Nat Cancer Inst 60:93–96, 1978
Issell BF, Crooke ST: Maytansine. Cancer Treat Rev
5:199–207, 1978
2012 Annual Meeting of the American Society of Clinical
Oncology (ASCO): Abstract LBA1.






















No comments:
Post a Comment