Judah Folmann once said, “There is a fine line between persistence and obstinacy. I have come to realize the key is to choose a problem that is worth persistent effort,” He followed it with: "Science goes where you imagine it."
Judah Folkman, MD
He spent his scientific lifetime imagining the concept of vascularity and cancer – or does cancer require additional blood supply to keep it humming? The answer to that question he got from his research was an unqualified yes and still is.
Perseverance... keeps honor bright: to have done, is to hang quite out of fashion, like a rusty nail in monumental mockery. ~William Shakespeare
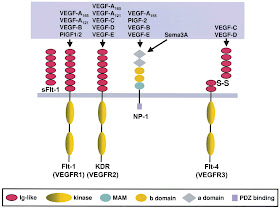
Cancer cells need blood supply to grow and prosper in their own right. Since the growth of cancer cells is progressive, they outstrip the normal blood supply of their host -an example may be colon cancer. So once the blood supply limit of the host , in the case of colon for instance, has been reached, the cancer cells have an ingenious regulator called VEGF Vascular Endothelial Growth Factor) abbreviated to “Veggef” that they liberate into the blood stream. (The VEGFs are Glycoproteins consisting of A-, B-, C-, D-, E- forms and Placenta Growth Factor (PLGF)).
The consequence of this product on the nearby tissues is that it actually promotes the growth of new blood vessels to form as new supply lines.
Imagine an army on a battle-field, the main concerns with advances into enemy-lines is the ability to maintain the supply chain. Lots of resources are committed to that prospect. The loss of supply leads inevitably to failure and possible eradication of the advancing infantry battalion.
Consequently a lot of strategies are developed to disrupt the supply-chains by either warring factions. And equally so, lot of time and energies are devoted to confusing the enemy (for the cancer the host patient is the enemy and vice versa) on where and how the supplies are being transported.
The effects of the VEGF expressed from cancer cells
Cancer needs the supply-chain to be constantly revamped and enhanced to feed its growing mass of tumor cells.
The VEGF released in the surrounding area gives birth to new unstable blood vessels which arborize to create a large network that can be seen on angiograms as a blush
The Tumor Blush
(due to the amount of blood supply) and on PET scans as a glow
PET Scans
(due to the rapid utilization of glucose by the cancer cells brought to it through the blood vessels). Sometimes in rapidly growing cancers outstrip even their own manufactured blood supply with the result that the central part of the cancer undergoes “necrosis” (Cell death).
Cancers on X-Rays will sometimes show a central echo (darkness) which indicates necrosis and is verified by pathological determination. Once the cancer cells have turned on the volume so to speak, their survival tactics change to the anaerobic method where they produce larger quantities of HIF-1 (Hypoxia Inducible Factor -1) to continue their growth patterns in face of reduced blood supply and lowered oxygen.
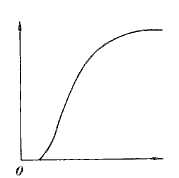
If you plot the growth pattern of the cancer cells they inevitably show a Gompertzian Growth Curve similar to population dynamics as pointed out by Laird in 1964. The Gompertzian function is defined by:
The initial phase is slow when the cancer cells are acquiring new blood supply, followed by a steep and rapid ascent which indicates a rapid doubling of tumor cell volume and that is followed by a slower rate of growth. This last phase has multivariate reasons for slowing down: the growth based on low or no oxygen, the growth limited by the pressures from adjoining tissues and central loss (necrosis) equaling the peripheral gain (new growth).
Q: So what has science done on the heals of Judah Folkman’s discovery?
A: Several compounds have been developed to thwart the VEGF released by the cancer cells.By far the most prominent of them is a compound called Avastin (Roche). Several studies have been undertaken to evaluate this drug.
Let’s look at the benefits and risks of this particular medication Avastin (Bevacizumab):
Avastin is approved for treatment of stage IV metastatic colo-rectal cancer, metastatic or recurrent unresectable non-squamous cell cancer of the lung and kidney (renal cell) cancer.
Metastatic Colo-Rectal Carcinoma (mCRC):
Based on the pooled data from large studies the average survival for good prognostic patients with mCRC is about 17-18 months. In randomized trials comparing Avastin+IFL chemotherapeutic regimen vs. the IFL regimen alone there is an improvement of 7-9 months (26-27 months). In patients previously treated with chemotherapy however, the benefits with Avastin were a modest 2.2 months when compared with equivalent chemotherapeutic regimen (FULFOX-4). The indications here are of some significance since the overall survival did increase lending to the veracity of the trial data. When overall survival increases in a given trial to the benefit of the drug under study, provided there has been no observer bias by front-loading of better performance patients versus those with poor physical states, younger patients compared to older patients, or some other confabulating circumstance then the trial data should be taken seriously. That is why the studies have to be considered in their entirety rather then just their conclusion.
However given the benefits one then has to look at the side effects related to the addition of the drug that potentially raises the survival time. Avastin is known to cause some side effects that occurred between 2-20% of the patient population with about an equal number (8.4-21%) of patients declining further treatment due to the complications.
These facts in cold hard currency of understanding suggest that a 7-9 month survival is associated with a 20% risk of complications that are severe and potentially life threatening. The foregoing clist is not complete but the most harsh ones are listed and are present irrespective of the organ site being treated. In other words whether it is colon or lung or kidney the treatment itself can induce such complications.
1. Gastrointestinal perforations and fistula formation. (Stomach or intestinal rupture followed by in cases with communication with other organs or other parts of the intestine and vagina, cervix, urinary bladder etc.
2. Delayed healing of surgical wounds.
3. Severe bleeding from the colon, lung, vagina nose or even in the brain.
4. Severe Hypertension associates with Heart damage and strokes.
5. Kidney failure.
6. Blood clots inside veins (partly also due to the cancer itself).
All of the afore mentioned complications can be monitored judiciously by the treating physician and successfully abrogated.
Non-Squamous Non-Small Cell Lung Cancer:
The E4599 trial showed both a (PFS) Progression Free Survival and (ORR) Overall Response Rate and modest (OS) Overall Survival improvement:
In (non-squamous) (NSCLC) Lung Cancer the benefits were even modest to a 2.3 month advance in overall survival when compared with standard chemotherapy of Taxol and Carboplatinum.
The squamous Cell Lung Cancers had a higher incidence of life-threatening pulmonary bleeding, hence patients with such diagnosis are excluded from anti VEGF therapy.
The cavitating effect secondary to necrosis due to therapy.
Kidney Cancer (Renal Cell Carcinoma):
In Kidney Cancer there was a significant progression free survival of 10.2 months compared with 5.4 months in patients treated with Interleukin-2 alone. However the overall survival was improved by 2 months only from 21 months for IL-2 alone to 23 months for IL-2+(A)vastin.
Breast Cancer:
The much discussed indication of Avastin against Breast Cancer came to a boil in December 2010 when the FDA Panel reversed itself and rejected offering Avastin to metastatic breast cancer patients citing “no advantage in survival but with significant side effects.” It appears based on the large studies, which support that contention. There was and still is a hue and cry about this rejection from both patient advocacy groups and certain physicians, yet on its own merit the argument appears sound and justified. The issue raised is one of Quality of Life Improvement in patients treated with Avastin versus the dangers of life-threatening Side Effects. Here the benefits clearly flatten the curve, so to speak and since there is no benefit in overall survival, the real truth based on data points available, show that Avastin in Breast cancer is a non-event.
Roche (+Genentech) is now seeking the use of Avastin in Ovarian Cancer as a first-line therapy advantage, claiming a delayed progression free survival and improved quality of life.
The data are to be presented soon from the OCEANS Trial which will set the stage for potential FDA approval. The preceding trials including ICON-7 seemed to suggest some PFS (Progression Free Survival) improvement but did not elaborate on OS (Overall Survival).
The E-2100 study (above data) that preceded the OCEANS trial (accrual completed, results are pending).
Again the FDA approval will lie heavily on the potential for increasing the overall survival and not on a statistical marmalade. Progression Free survival without overall survival is in the minds of some, equivalent to better supportive care. The impetus must be on OS rather than on response rate, PFS (Progression Free Survival) or DFS (Disease Free Survival) to win the approval of US Regulatory body concerned with costs. Roche’s pharma head Pascal Soriot said recently, "In Europe ... we are pretty confident because typically in Europe you can gain approval on the basis of good progression-free survival data. In the U.S., we still have to wait until the data are fully mature to see what the overall survival data look like and get a good sense for the FDA's response, but I have to say that what we have seen so far ... is making us relatively optimistic that we have a good chance in the U.S. as well.”
Notice I did not include Quality of Life in this circumstance and that is where some issues remain. After all what is the benefit of a quality of life improvement without improving the overall survival? Therein lies an expensive question with an equally expensive answer in monetary terms. And that begs the question, after all, what is life worth?
Completed and some Ongoing Trials:
| Trial ID | Trial acronym | Sponsor | Indication | ||
| NCT00262847 | GOG-0218 | NHI/GOG | Advanced ovarian cancer | ||
| NCT00483782 | Icon7 | Medical Research Council | Newly diagnosed ovarian cancer | ||
| NCT00434642 | Oceans | Roche | Platinum-sensitive recurrent ovarian cancer | ||
Speaking of costs a typical year-long therapy with Avastin costs around $100,000. There must be some bang for that expensive buck especially when vying for the public dollar.
So where do we stand or for that matter go? Is Avastin the Holy Grail that Judah Folkman once opined about? Or is it another expensive drug with marginal benefit? These questions at the outset when asked of the regulators and bean-counters seem to be answered in the negative but to that patient where the additional survival or quality of life survival is considered they are a life-saver. Emotions and arguments will sway public opinion both ways and ultimately the regulating body cognizant of the money issue will win as it always does. Anti VEGF therapy has proven Judah Folkman's dream. It is a "single" in baseball terms and four of those constitute a Home Run!
References:
Laird, A ,K, Brit J. of Cancer ,18(1964)490-50
Napoleone Ferrara, Robert S. Kerbel. Angiogenesis as a therapeutic target. Nature 438, 967-974 (15 December 2005)
Alberto Sobrero, Paolo Bruzzi. Bevacizumab Plus Fluorouracil: The Value of Being Part of a Developing Story. Journal of Clinical Oncology, Vol 23, No 16 (June 1), 2005: pp. 3660-3662.
Kohne CH, Cunningham D, Di Costanzo F et al. Clinical determinants of survival in patients with 5-fluorouracil-based treatment for metastatic colorectal cancer: results of a multivariate analysis of 3825 patients. Ann Oncol, 2002, 13, 308-317.
Kabbinavar F, Zurlo A, Irl C and Hurwitz H. Bevacizumab improves outcomes of patients with mCRC treated with IFL with or without bevacizumab independent of baseline risk. Proc ASCO, 24, 2006, Abstr. 3539
Sandler A, et al. A randomized phase II/III trial of paclitaxel plus carboplatin with or without bevacizumab in patients with advanced non-squamous non-small cell lung cancer.N Engl J Med. 2006 Dec 14;355(24):2542-50 E4599:
I. Rini, S. Halabi, JE. Rosenberg… Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206B. - Journal of Clinical Oncology, Vol 26, No 33 (November 20), 2008: pp. 5422-5428
GOG: ICON7 - A randomised, two arm, multi-centre Gynaecologic Cancer InterGroup phase III trial of adding bevacizumab to standard chemotherapy (carboplatin and paclitaxel) in first line treatment of patients with epithelial ovarian cancer


















No comments:
Post a Comment