Entropy is the natural state of the universe. All things suffer and decay with time. The landscapes change, the rocks are molded by the rain and wind, the green grass undergoes desertification and living beings die. Elements also undergo their natural recession. All radioactive elements undergo decay on a time clock, each to its own drumbeat.
Carbon-14 Cycle
Carbon Dating
For instance the Carbon-14, which is used extensively in dating natural materials has a half-life of 5736 years, which coincides with the human civilization. Each nuclear decay of this element ticks back 5736 years and configuring that loss, dates the material under study. That is how geologists are able to date various artifacts of history and the geological layers of rock formations, the Burgess Shale,
Burgess Shale
the age of King Tutankhamen, other mummies and even Lucy (Australopithicus afarensis circa 2.7-4 million years).
Lucy
History is replete with serendipitous expressions of discovery. Radioactivity falls in that category. In 1896 a Frenchman named Henri Becquerel discovered the radioactive emissions from Uranium while looking for phosphorescence in various elements. Ernst Rutherford first employed the term radioactivity in 1899 when he discovered the alpha and beta emissions from Uranium. In 1898 Nobel Laureates Marie Curie her husband Pierre and Henri Becquerel realized the complex nature of radioactive decay.
Henri Becquerel discovery of radiation
How does Carbon-14 do that? The sun is the source of radiation energy. As the cosmic rays collide with the Nitrogen-14 they liberate a proton and a Neutron. The Carbon captures the released Neutron to become Carbon-14. This is embedded in plant and animal life and gets buried in the soil ultimately decaying back to its Nitrogen form as its half-life clock ticks down the years. This instability expressed in the form of radioactive decay is related to the configuration of the Neutrons and Protons in the nucleus of the atom. It is balance that the atom tries to achieve between Neutrons (N) and the Protons (Z) by causing the radioactive decay and emission of particles. Thus release or capture of Neutrons is an attempt to achieve a nuclear balance. We, humans, exploit that imbalance to read time.
Radioactive Decay
“The common decay products are named after the first three letters of the Greek alphabet—alpha (a), beta (b), and gamma (g). In an alpha decay, a helium nucleus escapes from a nucleus. Alpha emission reduces the number of protons by two and also the number of neutrons in the nucleus by two. Beta decay can proceed either by emission of an electron, and an antineutrino or by emission of their antiparticles, a positron and a neutrino. Beta decay changes the number of protons and the number of neutrons in the nucleus by converting one into the other. Inverse beta decay involves the capture of an electron by a nucleus. In a gamma decay a high energy photon leaves the nucleus and allows the nucleus to achieve a more stable, lower energy configuration. Spontaneous fission of a large-mass nucleus into smaller-mass products is also a form of radioactivity.”
Uranium in Pitchblende
Interestingly a naturally found element in the soil is none other than uranium. In 1789 Martin Heinrich Klaproth discovered the silvery element after dissolving pitchblende in nitric acid and named it after the planet Uranus. The most common isotope of Uranium found in the earth is U-238. Although U-238 is fissile (breaks into smaller components) with high-energy neutrons, it however does not lend itself to a “chain-reaction.” U-238 can also capture its own Neutron and after two beta-decays convert into Plutonium-239. Pu-239 is highly fissile and used militarily and in commercial use for energy production in Nuclear Reactors. The military grade Uranium has to have between 3-5% of U-235 which has a lower half-life and is inherently more unstable and therefore given easily to fission. A “Depleted uranium is one that has less than 0.3% U-235 and therefore considered less fissile and is extremely dense. The DU is used in weaponry. The half-life of U-238 is an impressive 4.468 billion years.
Interestingly a naturally found element in the soil is none other than uranium. In 1789 Martin Heinrich Klaproth discovered the silvery element after dissolving pitchblende in nitric acid and named it after the planet Uranus. The most common isotope of Uranium found in the earth is U-238. Although U-238 is fissile (breaks into smaller components) with high-energy neutrons, it however does not lend itself to a “chain-reaction.” U-238 can also capture its own Neutron and after two beta-decays convert into Plutonium-239. Pu-239 is highly fissile and used militarily and in commercial use for energy production in Nuclear Reactors. The military grade Uranium has to have between 3-5% of U-235 which has a lower half-life and is inherently more unstable and therefore given easily to fission. A “Depleted uranium is one that has less than 0.3% U-235 and therefore considered less fissile and is extremely dense. The DU is used in weaponry. The half-life of U-238 is an impressive 4.468 billion years.
Uranium
Atom and its components
All radioactive elements are inherently unstable. The stability of the Element comes from when Neutrons (N) and the Protons (Z) are balanced. For example if the element has 6 Neutrons and 6 Protons, its nucleus is inherently stable. However any discrepancy between the two leads to radioactivity through decay by virtue of expulsion of the excess Proton/Neutron/Electron to achieve parity.
The emissions are in the form of “Alpha decay”. “Beta decay” or “Gamma decay.” Alpha decay occurs in heavy elements with 52 or greater Protons. The emitted particles are therefore heavy (Helium nucleus) and have limited penetrance beyond a thin layer of material.
The only way they can cause harm to humans and animals is if these are ingested and their radioactive decay occurs within embedded tissue causing genetic effects on the nearby cells. Beta decay occurs with smaller particles that can penetrate deeper.
Particle penetration
So external exposure to humans can lead to damage to the subsurface of the skin (subcutaneous layers). The penetration stops there due to material density. This occurs with all unstable elements.
Beta Decay
Nuclear Power Plants
Gamma particles are essentially electromagnetic radiation of the photons/electrons. The energy of the gamma particles determines the depth of penetration of the tissues. The expulsive emission from the nucleus if approximated with another unstable radioactive element that can capture the emission can lead to a contained fissile reaction with release of energy. Therein lies the mystery of the Nuclear Power Plant. The Nuclear Energy containment is nothing more than a sustained and contained nuclear reaction. Since the U-238 is inelastic and does not afford itself to a “chain-reaction” this very containment results in a sustained energy release and a contained fissile reaction.
Three Mile Island
What happens in an Nuclear Plant accident is if the fissile reaction becomes uncontained and it continues leading to a core meltdown resulting in escape of the radioactive material into the water used to cool the reactor and therefore expelled in the steam as in the TMI accident or contaminating the soil and underground water supply as in Chernobyl and Fukushima Daiichi disasters with release of radioactive byproducts of fission: iodine-131, cesium-134, cesium-137 and tellurium-132. Unfortunately the containment of the radioactivity is not linear, which means that distance does not necessarily diminish the radioactive intensity. 25 years after the Chernobyl Nuclear disaster the fall out continues to haunt humans with Leukemia and cancer related diseases.
Chernobyl aftermath
The radiation levels at Chernobyl at the time of accident were 200 times that found at Hiroshima and Nagasaki and based on atmospheric radioactivity monitoring in Scotland for a brief period following the accident the radioactivity level rose to 10,000 times normal values.
Hiroshima mushroom cloud
We call them disasters because of the effects on humans, animals and plants. Biological life forms live in a radioactive world. Radioactivity is in the atmosphere (cosmic radiation/solar radiation), in the soil and in the plants. Animals and humans who consume the plants and animals contain a minute fraction of these radioactive elements within their tissues. We are therefore constantly bombarded with radioactivity in our daily lives.
Radiation and the Human DNA
Our genetic makeup is designed to withstand this onslaught through a mechanism of DNA Mismatch repair mechanism. Overwhelming that mechanism leads to either cell death (apoptosis) or disease. For instance it is believed that the human DNA takes about 10,000 hits daily. In human cells, both normal metabolic activities and environmental factors such as Ultra Violet (UV) light and radiation can cause DNA damage, resulting in as many as 1 million individual molecular disruptions per cell per day. DNA damages in frequently dividing cells, because they give rise to mutations, are a prominent cause of cancer.
DNA damage and repair
In contrast, DNA damages in infrequently dividing cells are likely a prominent cause of aging due to the slow reactive damage inflicted upon these cells. (These hits are random based on radioactive decays from the soil and inherently assimilated natural radioactive substances incorporated in various tissues of our body and via solar radiation). It is true that the incorporated radioactive materials in the body have a long half-life i.e. their propensity to decay is extremely slow and therefore emissions of alpha, beta or gamma particles is rare. But it is there, maybe not constant but forever the bell that has the potential to toll. In the event of such disasters where there is a dramatic increase in the radioactivity in the environment the body can be overwhelmed with the onslaught and that can lead to human cellular framework disintegration and chaos.
The cancer cell is wayward cell that has lost all constraints and restraints programmed into it. This loss of restraint and constraint from normal function and form leads to unrestricted growth.
DNA single/double strand damage
Lets dig a little deeper into the cellular DNA both in the nucleus and the mitochondria. Imagine the DNA with its double helix held together by the phosphate sugars and linked together by extremely loose water-soluble hydroxyl (OH) bonds. Now imagine a stray bullet (in this case an escaped electron) dislodging one of those (OH) bonds and in so doing ricochets and hits the corresponding Nucleic acid. This attack leads to a compromised connection between the Nucleic acids Adenine with Thymine and Guanine with Cytosine and also disrupts the Nucleic Acid.
DNA repair Ligase
DNA Repair Mechanisms
The repair mechanism using default methods substitutes another Nucleic Acid and changes the entire message that that portion of the DNA represents. Now suppose that the damage occurred in the DNA where the gene was coding the antiproliferative (non-growth) activity of the cell, but with this dislodgement and disruption via change of the Nucleic Acid that code now no longer suppresses the growth activity. The cell is given the go-ahead signal to multiply without restriction and cancer results. That is akin to taking the foot of the brakes and jamming down the accelerator in the car. This disruptive action can also result in another portion of the DNA relegated to the Chromosome 9 in the long arm at position 21 which can lead to heart disease. So modulation of the DNA not only affects cancer formation it can lead to other disease as well. A disruption of an Alpha-1-Antitrypsin enzyme produced in the lung suddenly stops being produced due to the disrupted gene code by an accidental damage from a wayward electron can lad to Emphysema. The list goes on and on. The “out-of-nowhere” disease emergence, during the aging process in humans, has a causal basis in the environs and within us.
The energetic electron can also by virtue of affecting the hydroxyl (OH) group release nascent “free radicals” which essentially means that an electron was stripped out of the oxygen’s outer shell rendering it nascent and making it look for a source to feed itself on other easily derived electrons through disrupting cellular makeup. This frenzied feeding by the nascent oxygen to satisfy its appetite, leads to similar disruption of the proteins, DNA, RNA and all building blocks of the human cellular mechanism and can lead to aforementioned cell death or disease.
Inside mitochondria, reactive oxygen species (ROS), or free radicals, byproducts of the constant production of adenosine triphosphate (ATP) via oxidative phosphorylation, create a highly oxidative environment that is known to damage Mitochondrial DNA (mtDNA). Superoxide Desmutase is a critical enzyme that counters such activity in the cellular machinery and is present in both the mitochondria and cytoplasm of eukaryotic cells.
Radiation Poisoning
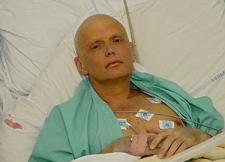
Acute Radiation Poisoning is a phenomenon when the body is espoused to a large amount of radioactivity. The former Soviet KGB agent Alexander Litvinenko was deliberately poisoned by polonium-210 and died soon thereafter.
Alexander Litveninko
It is estimated that 1Gy would enable such an event. This acute illness occurs as a result of damage to the fast-replicating cells such as those found in the skin (radiation burns), in the gastrointestinal tract (Nausea, vomiting and diarrhea), blood cells (Low white counts and platelet counts leading to infections and bleeding), Immune surveillance, tracheal burns and oral cavity (mouth) sores and bleeding.
I-131 and Graves Disease
The chronic nature of radiation damage is essentially a torrid affair between the radiation damage to the cellular DNA and the months and years it takes to create the cancerous tumor. The most common forms of cancer related disease include the Leukemias, Lymphomas and solid tumors such as Thyroid cancer. Thyroid cancer occurs by virtue of the I-131 finding a safe haven in the thyroid, since the thyroid gland is the pooled reservoir of all iodine in the body. I-131 emits 14 gammas and 6 beta decays and that is how it is used in the treatment of Graves Disease (Hyperthyroidism).
Graves Disease (Hyperthyroidism)
The beta decay causes 90% of the tissue damage to the thyroid gland leading to a hypothyroid state. Excess of the I-131 exposure when ingested through water or fish/animal food as in a nuclear fallout the I-131 after saturating the thyroid follicles finds other organs to bio-accumulate within like bone, liver, lung and kidneys where it creates cellular disruptive mischief.
Enlarged Thyroid & normal
Radiation as Therapy for Cancer
So if radiation is so bad for us then why do we use radiation therapy as a treatment? Before human knowledge had achieved its current state of understanding, self-made ”therapists” were touting the benefits of radioactive fluid lavages and enemas as panacea for all disease. It was another Nobel Laureate Hermann Joseph Muller who in 1927 discovered the genetic mutational effect caused by radioactivity. Only when the dangers became predictably known with this “therapy” did the FDA outlaw the practice. It is now known that bioaccumulated uranium in the tissues leads to Binucleated cells with micronuclei, Inhibition of cell cycle kinetics and proliferation; Sister chromatid induction, tumorigenic phenotype. Knowing the affinity of radiation to disrupt the cellular function and knowledge about the acceleration of the electron/photons via the electromagnetic wave came to reveal the benefits of radiation as a treatment methodology for cancer.
Linear Accelerators
Linear Accelerators were developed to accelerate electrons and then through focused beams these electrons/photons were directed at the source in this case a tumor to destroy the tumor without affecting the normal tissues. R. Wideroe built the first linear accelerator in 1928, accelerating positive ions to about 50 keV. This ability improved over time from the Cobalt-60 that was a byproduct created in the Nuclear Reactors and used for cancer therapy. Unfortunately it cast a large damaging penumbra effect on normal non-cancerous tissues and with innovation lead to the focused IMRT (Intensity Modulated Radiation Therapy) and IGRT (Image Guided Radiation Therapy) therapies available today. Now high-energy accelerators SLAC (3-km long at Stanford University, CA) are capable of harnessing more energetic photons/electrons (Energies up to 50GeV) to deliver precise doses to the embedded tumors. Meanwhile newer modalities like Proton Beam therapy that use massive particles such as protons or alpha particles to deposit the bulk of their energy in the tumor bed are being used in cancer care. (This maximal accumulated dose before rapid decline is called the Bragg Peak.
Bragg Peak
Using Bragg peak helps direct the radiation energy precisely to the tumor without exposing the normal intervening tissue).
Boron Neutron Capture Therapy (BNCT) is under development, In BNCT; boron is synthesized into compounds that are selectively taken up by cancerous cells in the brain and not by healthy ones. It is being utilized in Brain tumors like Glioblastoma Multiformi with greater success.
BNCT
Do we need protection against radioactivity? The answer is: Absolutely (as much as possible). Are we safe from all radioactivity? The answer here is no! We are as mentioned, constantly exposed to radioactivity be it in the form of cosmic rays or diagnostic X-Rays we do get it in our daily lives. Flying for instant at 35,000 feet in an airliner exposes one to a higher level of cosmic radiation then at the sea level due to some protection afforded by the Ozone layer and other particulate matter suspended in the atmosphere. So we cannot exist without some exposure, but we certainly ought to be cognizant of sources of radiation that we can knowingly avoid.
Radon and Lung Cancer
Another little known source but highly toxic to humans is the Radon (Rn86) present in the soil. Radon is an odorless gas when present in the soil can accumulate in closed, unventilated spaces like the basements and in the attics. A way to detect that is to vacuum the spaces and use a Geiger counter to determine the background radioactivity. If there is increased radioactivity determined then appropriate ventilation should be considered such as attic fans and basement windows. “Air the house periodically.” Radon has been estimated to cause about 30,000 cases of lung cancer per year.
Radiation Hazards
Examples of naturally occurring radioactive hazards include the following:
Radiation source | Comments | mSv/yr | mrem/yr |
Natural sources | |||
indoor radon | due to seepage of 222Rn from ground | 2.0 | 200 |
radionuclides in body | primarily 40K and 238U progeny | 0.39 | 39 |
terrestrial radiation | due to gamma-ray emitters in ground | 0.28 | 28 |
cosmic rays | roughly doubles for 2000 m gain in elevation | 0.27 | 27 |
cosmogenic | especially 14C | 0.01 | 1 |
total (rounded) | 3.0 | 300 | |
Medical sources | |||
Diagnostic x-rays | excludes dental examinations | 0.39 | 39 |
Medical treatments | radionuclides used in diagnosis (only) | 0.14 | 14 |
total | 0.53 | 53 | |
Other | |||
consumer products | primarily drinking water, building materials | 0.1 | 10 |
occupational | averaged over entire US population | 0.01 | 1 |
nuclear fuel cycle | does not include potential reactor accidents | 0.0005 | 0.05 |
TOTAL (rounded) | 3.6 | 360 |
Hamlet says to Horatio: “There are more things in heaven and earth, Horatio, than are dreamt of in your philosophy.”
The last question worth asking is what will happen to Japan’s West Coast after the March 11, 2011 Earthquake near Tahoka and the ensuing Tsunami caused the Fukushima Daiichi nuclear meltdown with ongoing aftershocks near Honshu. If Chernobyl history is a reference point then a 40-year ecological disaster remains in effect. The 100 km area around ground zero will remain radioactive making it uninhabitable for humans. Modern techniques of ion exchange from the soil and ground water may speed the process and help the ecology recover faster maybe. The coastline of the Northeastern Japan has changed for this and the next generation of Japanese forever.
Our lives are lived in the fear of death, since death taunts us at every step of the way. Human lives are lived in the quiet desperation of that and other imagined fear. Understanding, avoiding and preventing are the steps to a fruitful life. Engaging life and its predicates with an open mind and enjoying every valuable moment is a life well lived. The anatomy of that conjugate thought is an aspiration devoutly to be wished.
References:
Lodish H, Berk A, Matsudaira P, Kaiser CA, Krieger M, Scott MP, Zipursky SL, Darnell J. (2004). Molecular Biology of the Cell, p963. WH Freeman: New York, NY. 5th ed
Campisi J, d'Adda di Fagagna F (2007). "Cellular senescence: when bad things happen to good cells.". Rev Mol Cell Biol. 8 (9): 729–40
Best,BP (2009). "Nuclear DNA damage as a direct cause of aging" (PDF). Rejuvenation Research 12 (3): 199–208
S. Craft, A. W. Abu-Qare, M. M. Flaherty, M. C. Garofolo, H. L. Rincavage, M. B. Abou-Donia (2004). "Depleted and natural uranium: chemistry and toxicological effects". Journal of Toxicology and Environmental Health Part B: Critical Reviews 7 (4): 297–317.
Proton beam therapy" W P Levin, H Kooy, J S Loeffler and T F DeLaney British Journal of Cancer (2005) 93, 849–854
Zamenhof, R.G., Murray, B.W., Brownell, G.L., Wellum, G.R., Tolpin, E.I., "Boron Neutron Capture Therapy for the Treatment of Cerebral Gliomas. 1: Theoretical Evaluation of the efficacy of Various neutron Beams," Med. Phys., 2: 47-60, (1975).
Barth, R.F., Solloway, A.H., Fairchild, R.G., "Boron Neutron Capture Therapy of Cancer", Cancer Res., 50:1061-1070, (1990)
http://www.lbl.gov/abc/wallchart/guide.html


































No comments:
Post a Comment